
Commentary: Addressing the Ethical Challenges of Noninvasive Prenatal Testing
Advancements in genetics have changed the landscape of prenatal care in just the past five years, with a technology popularly known as noninvasive prenatal testing (NIPT). Pregnant women now have the opportunity to learn an unprecedented amount of information, such as the chance that their future child might have Down syndrome, from a simple blood draw. But this information, which is useful but not diagnostic, can easily be misunderstood – as health care providers, families and others are learning quickly.
Actually, everything is happening quickly right now in the world of prenatal genetics. It has to, because the technology is moving at a pace that’s “unprecedented in the history of prenatal care.” I’ve been researching the bioethical and social issues surrounding NIPT since it first appeared on the horizon, about five years ago, and I’m discovering that it taps into huge areas of change and controversy in medicine and society.
A Little Backstory
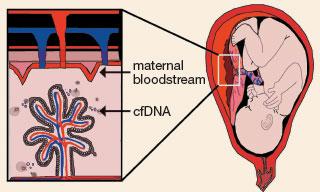 Circulating free DNA shedding into the maternal bloodstream
Circulating free DNA shedding into the maternal bloodstream
Just under 20 years ago, a young scientist named Dennis Lo discovered fragments of fetal DNA circulating in pregnant women’s blood (now known as “circulating free DNA,” or cfDNA). A year later, he suggested that this genetic material might enable what’s been called the “Holy Grail” of prenatal testing: noninvasive prenatal diagnosis, the fabled ability to definitively diagnose fetal conditions without an invasive procedure like amniocentesis. It took 10 years for the technology of DNA sequencing to advance enough that this concept could take shape. But in 2008 two teams of scientists – Dennis Lo’s team at the Chinese University of Hong Kong, and Stephen Quake’s team at Stanford, within weeks of one another – published proof that cfDNA could provide accurate information about genetic conditions in the developing fetus.
If we were talking about a new drug, here’s where things would slow down a bit. The Food and Drug Administration (FDA) regulatory process would kick in, requiring animal testing, human safety trials and large-scale studies of effectiveness. However, most genetic tests fall into a well-known FDA loophole: laboratory-developed tests (LDTs). The LDT category was designed to enable labs to use their own simple tests with a limited population without a long approval process (think: a hospital lab’s test to measure the amount of sodium in a patient’s blood sample). But LDTs have now proliferated to include multimillion-dollar products, with companies exploiting the regulatory loophole to enter the market without prior FDA approval or postmarket monitoring. The FDA is pushing to close the LDT loophole, but as with any government action, this may take years.
Back to our story: without the need for FDA oversight, NIPT hit the American market in less than three years, and is now available nearly worldwide.
Missing Details Cause Ethical Challenges
However, it has taken time for some important details about this new technology to reach health care providers and patients. The most important caveat that people often miss is that we aren’t talking about diagnosis. While it’s possible that we’ll get there eventually, the technology that we have now is a very good screening test for a few conditions. Yet as clinics started using these cfDNA tests, it became clear that the test performance statistics commercial labs were publicizing (usually some version of “99% accurate!”) were either best-case scenarios or carefully vague to deflect attention from the test’s limitations. Powerful marketing campaigns to both providers and patients, combined with limited published information and fast-moving technology, meant that many providers weren’t well informed about the tests – and some women were making wrenching decisions about their pregnancies based on incorrect assumptions.
One woman, Stacie Chapman, shared her haunting story with reporter Beth Daley, recalling that she nearly ended her pregnancy based on a false-positive cfDNA screen, which her doctor mischaracterized as near-diagnostic. Other providers have reported cases of women terminating pregnancies without confirming their cfDNA result, though all professional guidelines strongly recommend diagnostic confirmation.
Another caveat about prenatal cfDNA screening – really, about all prenatal testing – is that even when results are accurate, we don’t necessarily know what they mean. Sure, some conditions like Down syndrome are well understood, but even then, outcomes and abilities vary widely. This isn’t just because of biology, but also because access to support and interventions is so variable. And for many other fetal conditions, it’s very difficult to predict how having a genetic condition will really play out in the future child.
Education Is Crucial
This is why it’s so crucial to educate providers and patients about both the tests and the conditions they test for. The good news is that many efforts to do this are mobilizing. The Perinatal Quality Foundation (led by UC San Francisco physician Mary Norton) will soon release Genetic Education Modules for both providers and patients. They’ve also joined with the National Society of Genetic Counselors to make available the NIPT/cfDNA Screening Predictive Value Calculator. This easy-to-use, free online tool takes into account a variety of risk factors (the mother’s age, ultrasound results, serum screening and so on) to help providers determine the chances that a cfDNA screening test result is a true positive or true negative. Other organizations, such as the American College of Obstetricians and Gynecologists (ACOG), the Genetic Support Foundation, lettercase.org and the National Center for Prenatal and Postnatal Resources, have also launched valuable resources to educate providers and parents about prenatal cfDNA and genetic conditions.
But there’s much more to be done, especially since the technology isn’t likely to slow down (noninvasive prenatal whole-genome sequencing is probably coming soon). Both in the US and internationally, we’re building increased collaboration among clinicians, patient advocates, bioethicists and many others, to create better research, quality education and responsible policy.
 Marsha Michie, PhD, is an anthropologist and empirical bioethicist who studies social and ethical issues in genetics, including moral and religious perspectives. She is part of the UCSF Bioethics team and has conducted research on prenatal testing, on families of children with genetic disorders, on the understandings and self-perceptions of genetic research participants and on individual and community religious identities.
Marsha Michie, PhD, is an anthropologist and empirical bioethicist who studies social and ethical issues in genetics, including moral and religious perspectives. She is part of the UCSF Bioethics team and has conducted research on prenatal testing, on families of children with genetic disorders, on the understandings and self-perceptions of genetic research participants and on individual and community religious identities.



